Decentralized clinical trials (DCTs) are changing research. Unlike their conventional counterparts—which often require participants to travel to study sites—DCTs leverage digital clinical trial technologies to support remote participation.
This paradigm shift holds immense promise, offering greater accessibility, diversity, and efficiency in clinical trials.
According to the Federal Drug Administration (FDA), “a DCT refers to a clinical trial where some 20 or all of the trial-related activities occur at locations other than traditional clinical trial sites.”
In this article, we delve into the transformative potential of decentralized trials, exploring their benefits and challenges. We’ll also look at the future they hold for medical research and patient care.
To learn more about decentralized clinical trials and what approach is right for you, download our guide to decentralized clinical trials.
What Are Decentralized Clinical Trials?
A decentralized clinical trial (DCT) is a trial that does not take place at one central location. There are two types of decentralized clinical trials: fully remote and hybrid. Each type of DCT has varied levels of decentralization which could include at-home visits, remote electronic consent (eConsent), or other remote elements.
Why Are Decentralized Clinical Trials Important?
DCTs are revolutionizing clinical research. This new approach to trials offers an efficient, cost-effective, and resilient way to conduct a clinical study..
Remote trials are also more accessible and patient-centric, allowing sponsors to attract a greater variety of patients.
Decentralized clinical trials are important because they:
- Extend clinical trial access to underserved communities
- Provide participants with options to reduce burden
- Eliminate costs and provide operational efficiencies
Explore our solutions to make your clinical trials more accessible and efficient.
The Benefits of Decentralized Clinical Trials
The decentralized clinical trials market is growing. More sponsors are recognizing the benefits of these innovative approaches.
When they’re done right, they can reduce the administrative burden on site and study staff. DCTs can also increase diversity, boost retention, and support nimble studies.
Enhanced participant access and convenience
By removing the need for brick-and-mortar sites, a decentralized clinical trial allows patients to engage online, at home, through local labs, or at healthcare sites.
DCTs are leveraging novel technologies to improve access and convenience for trial participants outside of the conventional clinical setting.
Discover how our clinical trial solutions enhance participant access and convenience.
Access to real-world insights
By collecting data in participants’ home environments, DCTs provide a more accurate reflection of how treatments perform in real-world settings.
This real-world evidence (RWE) can complement traditional trial data, offering valuable insights into treatment effectiveness and safety
Increased diversity in participant pools
Travel can be a big burden for clinical trial participants. In-person site visits may require long commutes, childcare expenses, or time off work.
Sponsors can reach previously unattainable patients by reducing—or even eliminating—the need for in-person visits.
DCTs make it easier for participants from various socioeconomic settings to participate in research. This shift helps sponsors apply regulatory guidance to have greater diversity among participants.
Improved patient retention rates
Decentralized trials mean reduced travel since they often use eConsent and other remote methods of participation.
It can also be simpler to offer online translation services in a decentralized trial, since these can be done online rather than on-site. These kinds of options can make it easier for participants to stay in the trial until its end.
Decentralized methods can optimize participant experience and reduce caregiver burden, leading to higher retention rates. This is especially important when facilitating long-term research on diseases affecting populations with limited mobility.
Learn three best practices for implementing eConsent to ensure adoption and aid retention.
Faster recruitment timelines
One study found trials that adopted patient-centric technology were more efficient at recruiting trial participants.
The study, which examined nearly 4,000 phase II and III trials, found that patient-centric trials took an average of four months to recruit 100 patients, versus seven months for traditional trials.
Cost-effectiveness, data accuracy, and operational efficiency
In traditional trials, monitors need to be on-site to collect, share, and review data from participants. But remote electronic data collection has reduced, and in some cases eliminated, this role.
Furthermore, remote data collection has rendered multiple research sites no longer necessary for some trials, reducing site-specific inconsistencies and variability of data reports. In turn, there is a reduced need for institutional review boards (IRBs) to validate trial results.
Increased likelihood of going to market
The study mentioned above found that drugs developed with patient-centric, decentralized technology were 19% more likely to go to market than drugs developed using traditional in-clinic methods.
The numbers were even more staggering for oncology trials, where trials were 32% more successful at getting to market using patient-facing, adaptive technology.

The Challenges of Decentralized Clinical Trials
While there are many benefits to DCTs, some challenges persist. Since the world of clinical research is slow to adopt new approaches, these challenges can slow down the adoption of remote trials.
To learn more about the challenges and benefits of decentralized clinical trials, download our guide to decentralized clinical trials.
Technological and connectivity barriers
Not everyone has access to the necessary technology needed to make DCTs work.
A large percentage of the world population does not have access to WiFi, cellular, or personal mobile devices. Others may have access to technology but don’t have the knowledge or confidence to fully use it.
Data security and privacy concerns
Clinical research professionals are rightly concerned about protecting sensitive health data. Since remote trials rely on wearables, apps, and web-based interactions, there are cybersecurity risks.
While all trials come with some data security risks, DCTs face greater data protection challenges. Therefore, cyber security must be taken to protect clinical trial data, personal data, and information.
Any solution needs to adhere to global regulatory and compliance certifications including FDA CFR Part 11, GDPR (EU), ICH GCP (HIPAA, US), ISO 27001, and ISO 9001.
Learn more about the appropriate data protection steps any DCT vendor should take in this clinical data security infographic.
Technology adoption
With provisioned devices, technology adoption can be challenging.
Instead, with “bring your own device” (BYOD) technologies, participants can overcome common clinical trial barriers, such as technical proficiency. Operating on apps and devices that patients already know how to use can increase engagement and help minimize confusion, ultimately reducing drop-out rates.
In this DCT technology success story, learn how Swing Therapeutics received 98 percent patient compliance with a “bring your own device” CDMS/EDC and ePRO/eCOA solution, ultimately receiving FDA approval.
Management of decentralized data
When conducting a decentralized trial, sponsors may need to manage multiple sources of data collection. For example, digital health technologies (DHTs) have broadened the types of data that can be obtained remotely from participants.
Sponsors need a data management plan (DMP) to show regulatory bodies how they will handle this incoming data.
According to the FDA, a DMP should include:
- Data origin and data flow from all sources to the sponsor
- Methods used for remote data acquisition from trial participants, staff, and service providers
- A list of data collection, handling, and management vendors
In this decentralized clinical data webinar, learn how Roche Diagnostics standardizes data collection to better manage their clinical trial data.

How Do Decentralized Clinical Trials Work?
There are two types of decentralized clinical trials: fully remote and hybrid. Each type works differently.
Fully remote decentralized clinical trials
In fully decentralized trials, all activities take place away from a traditional clinical site. Participants might take part in trial-related activities at their homes or at nearby healthcare facilities.
Another key element of decentralized trials is remote data collection. This includes eConsent, home nurse visits for vital signs, ePRO, or telemedicine visits.
Fully decentralized trials may be appropriate for investigational products (IPs) that:
- Are simple to administer or use
- Have well-defined safety profiles
- Don’t involve complex health assessments
For fully remote trials the typical trial experience may include:
- Electronic consent for remote informed consent and a participant portal
- Home nurse visits for vital signs, samples, and other activities
- Electronic patient reported outcomes (ePRO) to record daily outcomes and adverse events
- Telephone calls or telemedicine visits to check on participant progress
Hybrid decentralized clinical trials
In hybrid DCTs, participants visit traditional clinical trial sites for some trial activities. Some activities, however, happen at home or at a nearby healthcare facility.
Hybrid decentralized trials may be appropriate in trials where:
- Administration of an IP needs to be performed at a clinical trial site
- Complex medical assessments need to be done in-person
- Follow-up assessments can be performed remotely through ePRO, telehealth or in-home visits, or by local healthcare providers
For hybrid trials the typical trial experience may include:
- On-site screening
- On-site consent or electronic consent (eConsent)
- Electronic patient-reported outcomes (ePRO)
- Telemedicine
Read our guide on decentralized clinical trial types to learn which design is right for your trial.
Technology for Decentralized Clinical Trials
Success relies heavily on advanced decentralized clinical trials technology.
Patient-facing technologies are playing an increasingly important role in the future of full remote and hybrid decentralized trials. They are enabling remote patients to participate in clinical trials where they haven’t been able to before.
Common patient-facing for technologies decentralized trial elements include:
- Electronic Consent (eConsent)
- Electronic Patient Reported Outcomes (ePRO)
- Telemedicine
- Online participant portals
Conducting a decentralized trial isn’t just about having the right patient-facing solutions. To be truly successful, the trial needs a unified CDMS/EDC platform that can also support data quality, privacy, and compliance.
Additional solutions for decentralized trials include:
- Clinical Data Management System (CDMS) or Electronic Data Capture (EDC)
- Randomization and Trial Supply Management (RTSM)
- Electronic clinical outcome assessments (eCOA)
- Risk-based quality management (RBQM)
- Electronic trial master file (eTMF)
- Clinical trial management system (CTMS)
- Imaging
- Telemedicine
- Wearables
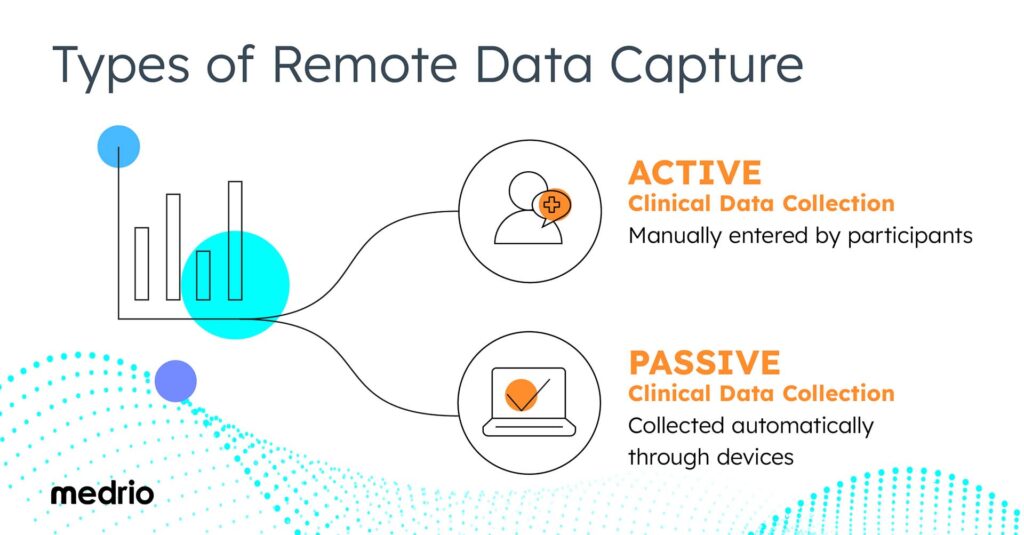
Types of Remote Clinical Data Capture
DCTs can include both active and passive clinical data collection. Both types of data collection offer benefits and add valuable insights to a clinical trial.
Sponsors should build protocols that collect data remotely in the most efficient and accurate way possible.
Learn how to seamlessly collect and manage high quality clinical trial data.
Active Clinical Data Collection
Active data collection means that participants must manually enter data directly. Participants may enter data through a link sent via email or text link or through an app on their mobile device.
Passive Clinical Data Collection
Passive data collection occurs when data is collected automatically. Typically this automatic data collection takes place through wearable devices.
How to Implement Decentralized Clinical Trials
Conducting a decentralized trial requires careful planning and thoughtful trial design. There are many factors to consider.
When designing a DCT, sponsors need to:
- Define trial objectives and endpoints.
- Select appropriate technologies, such as wearables or cloud-based apps.
- Design participant-friendly protocols.
- Decide whether participants will use their own mobile devices.
- Recruit and consent participants remotely, using eConsent.
- Conduct remote visits and data collection.
- Ensure data security and regulatory compliance.
Regulatory Compliance in Decentralized Trials
Since the pandemic, many regulatory agencies have issued guidance on conducting remote trials. In May 2023, the FDA released a draft DCT guidance document.
According to the FDA, “Specific issues related to the feasibility, design, implementation, or analysis of a DCT should be discussed early with the relevant FDA review divisions.”
When communicating with regulatory bodies about plans to decentralize a trial, sponsors should include information about:
- Local healthcare facility, healthcare provider, and lab usage
- Plans for telehealth visits
- Visits to trial participants’ homes
- Direct distribution of investigational product (IP) to trial participants

Decentralized Clinical Trial Strategies That Work
Sponsors can use technology to decentralize many aspects of a trial. Sponsors can use technology to:
- Manage eConsent, including version tracking
- Capture and store reports from remote trial personnel, HCPs, and labs
- Manage electronic case report forms (eCRFs)
- Track IPs shipped directly to participants
Discover what strategies will work for you in our guide to decentralized clinical trials.
Why Decentralization Is the Future of Clinical Trials
Decentralization holds the promise of revolutionizing clinical research. When done right, it can democratize access, improve diversity, and boost efficiency.
Embracing a decentralized approach paves the way for a future where healthcare is more inclusive, agile, and patient-centric. DCTs will continue to evolve and could eventually become the default approach in clinical research.
Streamline Your Decentralized Clinical Trials With Medrio
When your decentralized trial is built on the right technology foundation, anything is possible. Medio’s unified platform is your gateway to decentralized trials.
Decentralized trials aren’t one-size-fits-all. That’s why we’ve developed our solutions to be so flexible and interoperable.
Our solutions are interoperable and can be configured based on your needs. Whether you’re looking to kick off a hybrid study, or you’re ready to go fully remote, our solutions can support your research needs.
Solutions offered by Medrio include:
- CDMS/EDC: Robust clinical data management system that combines an intuitive user interface, seamless online and offline data capture capabilities, and comprehensive data management for clinical trials.
- eCOA/ePRO: Support DCTs with convenient and accurate outcome reporting, enhancing data quality and study efficiency.
- eConsent: Streamline all aspects of the informed consent process and enable participants to consent from anywhere..
- RTSM: Create seamless supply chain operations by confirming participant eligibility, ensuring delivery right to their home as needed, and distributing dosing instructions and schedules.
- Services: Receive the support you need with internal DCT expertise to meet your clinical trial data management needs.
Want to know more about our solutions? Visit the Medrio solution page.
FAQs About Decentralized Clinical Trials
Patients play a central role in DCTs as active participants. Their responsibilities include providing informed consent, adhering to study protocols, participating in remote visits, and accurately reporting their health data using digital technologies. They may also be asked to keep a digital diary or use a wearable device, such as a smartwatch.
Ensure data integrity in your decentralized trial by choosing a reliable digital platform with built-in security features. Train staff and participants on how to use digital tools correctly to minimize errors in data entry and reporting. Use real-time edits checks to find data entry issues early. Use real-time monitoring to ensure participant compliance.
All trials can support some decentralized elements but not all clinical trials can be fully decentralized. Trials that are particularly well-suited for decentralization are those that require longitudinal monitoring, involve chronic conditions, or target rare diseases. Trials relying on patient-reported outcomes, real-world evidence, or large sample sizes also benefit greatly from decentralized methods.



