Decentralized Clinical Trials (DCT)
The Future Platform for Clinical Research
Our integrated decentralized clinical trial platform includes eConsent, ePRO, RTSM, and DDC. It’s your gateway to more efficient decentralized trials.
Whether you want to dip your toe in the decentralized waters with a hybrid study, or you’re ready to go full-on virtual, our DCT solutions can get you there. And we do it all with flexible technology that improves your data quality, reduces patient burden, and supports a bring-your-own-device model.

Everyone is Talking About Decentralized Clinical Trials
The steadily rising use of telehealth combined with the difficulty of getting patients to sites during the pandemic are just a couple of reasons why decentralized clinical trials are growing in popularity.
Here are a few other benefits of a decentralized approach:
- Increased patient engagement
- Cost savings to you
- Improved compliance and better data accuracy
- Expanded recruitment potential with remote patient access
- Reduced patient drop-out
We have experts on staff ready to review your protocol and let you know if decentralization is right for you.
A Symphony of Integrated Decentralized Solutions
Decentralized trials aren’t a one-size-fits-all solution. That’s why we’ve developed our DCT platform to be flexible and interoperable.
Our unified DCT solutions can decentralize your trial with a combination of configurable solutions based on your needs. We’re so flexible, you can even mix-and-match with other vendors. Once you see how user-friendly our unified platform is, you won’t want to go back to the cumbersome technology you’re used to.
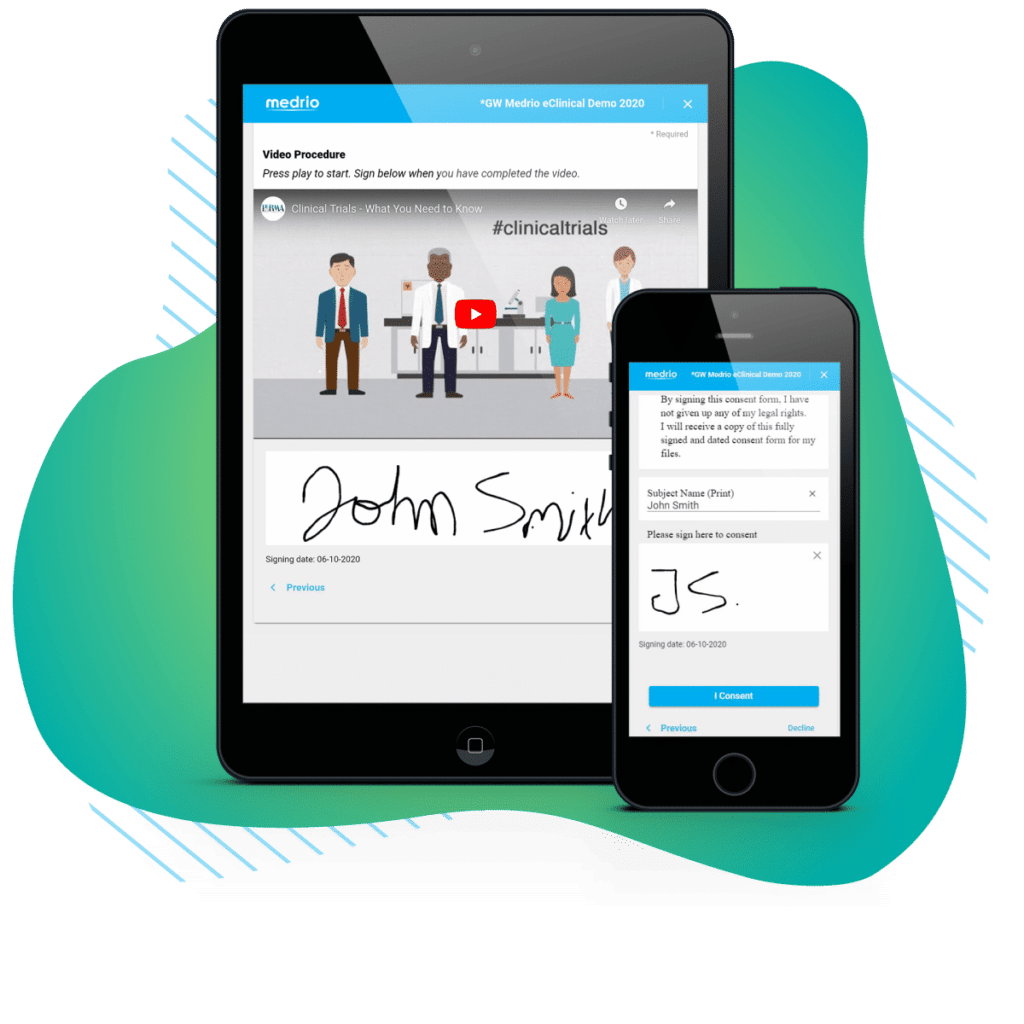
More than 50% of patients initially identified for a study will drop out because they fail to consent. eConsent gives you the tools to make the consent process far less painful for the patient—and easier for you to update and monitor. Our in-clinic and remote workflows support any trial type and help you consent patients where they feel most comfortable. Go a step further by enriching your forms with videos, FAQs, or quizzes to guarantee comprehension and reduce patient drop-out.
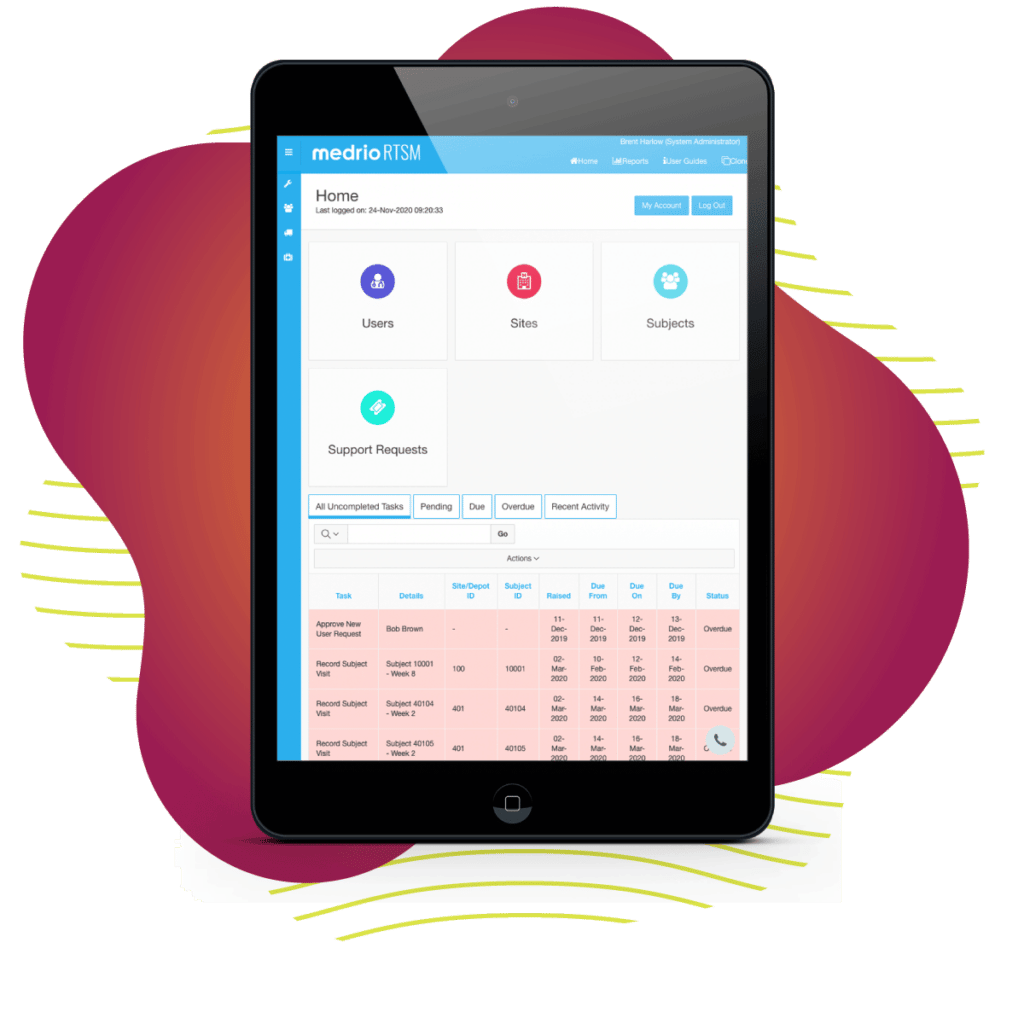
We know randomization can make or break a study. What if a single solution could help you reduce site overages by 20 – 40% while increasing patient compliance and meeting all of your randomization needs? It would be a game-changer, and we’re here to tell you—we’ve changed the game.
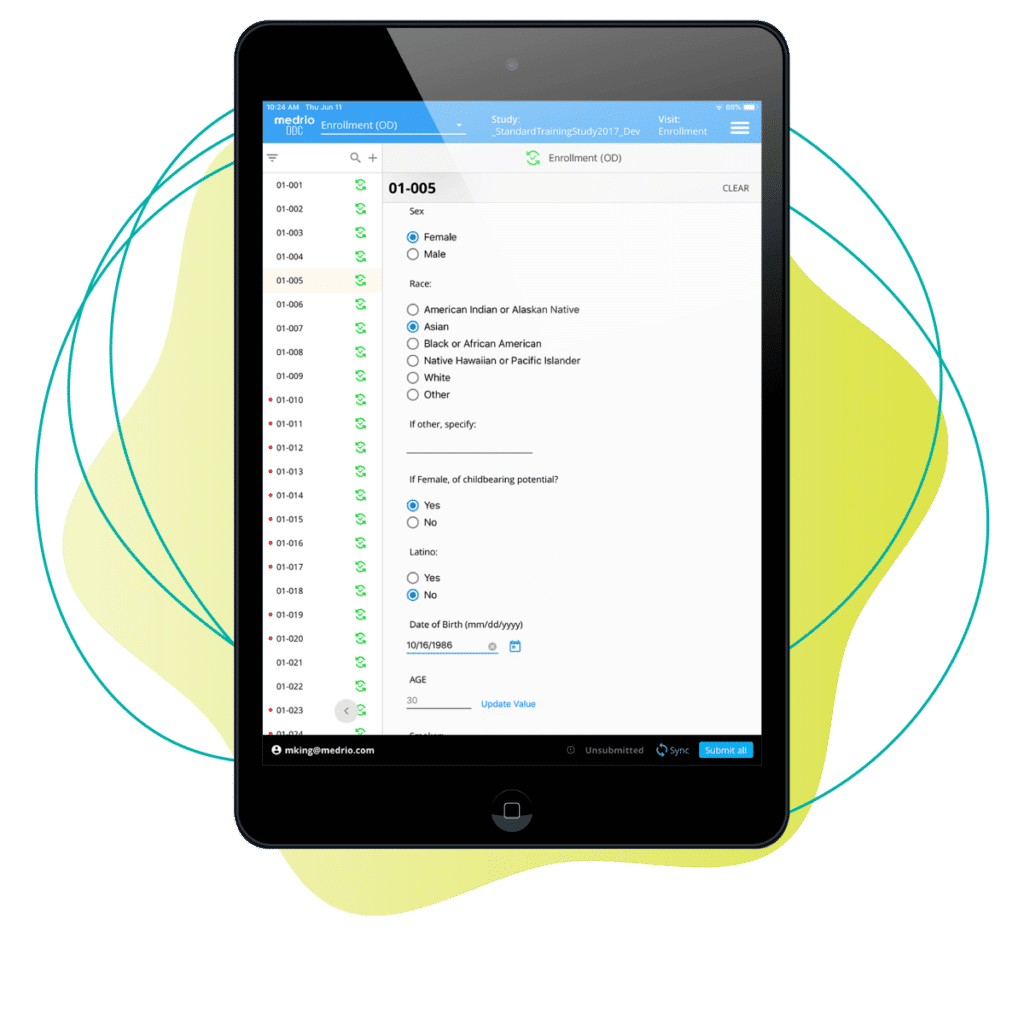
No connection? No problem. Using mobile tablets, your team can capture study data at the source whether they’re online or off, and it automatically syncs to your EDC as soon as that connection is restored.

Don’t Decentralize Alone
When you’re ready to try out some decentralized solutions, we’ll be here. Whether you want to start slowly by introducing eConsent into your next study or if you want to plan a fully decentralized trial, our support team will get you started and stay with you through the end.
Our scientific and technical experts—who can translate technical speak into plain language—will walk you through study builds, starts, mid-study changes, and any challenge (or minor hiccup) you might face. If you have a question, call us anytime, day or night.
Ready to Get Started?
We’ll show you how easy it is to manage your decentralized clinical trials with our unified platform.