According to a 2019 CISCRP study, 85% of the general public are willing to participate in clinical trial research. Yet, recruitment continues to plague the industry as one of the most costly and timely parts of conducting a trial.
Even worse, once studies are underway, teams still need to worry about patient retention in clinical trials and patient drop-out and adherence.
Patient withdrawal or non-adherence can stem from a variety of factors including geographical and financial constraints, fear and anxiety, or feeling a lack of appreciation. Whatever the specific reason, the underlying problem is the same:
Patients lack critical information about how to find a trial, what the trial will involve, and how the trial will affect their current well-being.
These lapses in information exchanges may result in additional recruitment for your sites or result in too many dropouts that make your trial underpowered.
Patient education is helping clinical teams strengthen their recruitment and retention efforts by creating healthy information and data exchanges.
Understanding the importance of patient education throughout the entire trial lifecycle helps to examine the unintended consequences of uninformed patients and then look at how education efforts are bridging the gap for clinical trial recruitment and retention.
Patient Education’s Impact on Recruitment
Patient recruitment continues to plague researchers, with the average trial extending recruitment timelines by up to 71%. And each day a trial is delayed due to recruitment, it can end up costing sponsors between $600,000 – $8 million.
But, why does recruitment continue to be so cumbersome for researchers, sites, and patients alike? For decades, clinical trial recruitment largely relied on word-of-mouth or paper-based processes.
Also Read: ePRO vs Paper Strategies in Clinical Trials
Patients would traditionally hear about available studies from their primary physician, who might have limited details on the specifics of the trial and how it would impact their long-term health. Then they might be asked to travel upwards of 2 hours to the nearest site to begin a screening process they knew very little about.
But as the global pandemic forced the clinical research industry to start replacing paper processes and focus on patient-centric technology, it still begs the question—why does recruitment continue to suffer?
The answer stems from a few reasons:
- A majority of the general public has indicated they’d be willing to participate in clinical research, but only 38% were actually asked to participate.
- Half of the general population claim they are unaware of clinical trials available to them and 57% do not recall ever seeing advertising for clinical trials.
- Only one in three individuals claimed they felt “confident” (20%) or “very confident” (10%) that they could find or identify a clinical trial if they wanted to participate.
In short: lack of clinical trial awareness continues to be one of the biggest barriers to patient entry in clinical research.
Patient education and awareness are critical in the recruitment stage because eligible patients still aren’t aware of where to find available trials, what they involve, or how to provide informed consent. And studies continue to show us that when patients don’t understand trials, they won’t participate in them.
The Damaging Impact of Uninformed Patients on Study Success
Beyond recruitment, patient education plays an important role in retention and overall patient satisfaction with a research study. With traditional trial operations, patients face a rising number of burdens for participation.
From time commitments, travel obligations, informed consent, and treatment expectations—it’s easy to understand why patients feel motivated to withdraw from a study.
A survey comparing the experience of patients who dropped out of a trial vs. those that completed a trial found striking differences in their level of understanding what was expected of them:
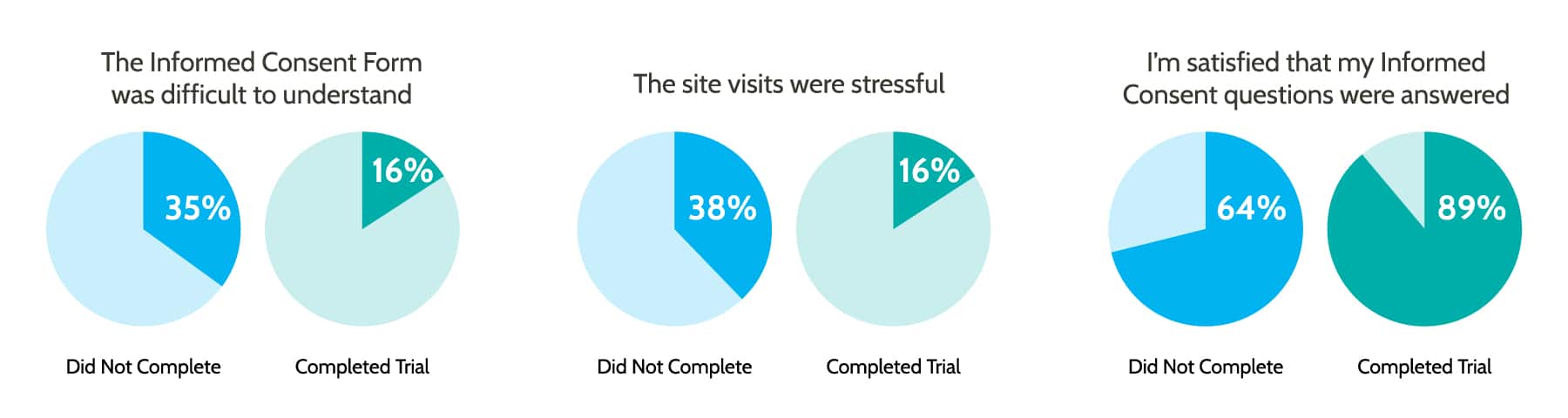
Educating patients throughout the process can help dispel fears and answer questions that strengthen adherence and compliance. The more clearly a disease or treatment is understood, the more likely it is that your patient will be comfortable with their care and adhere to the necessary treatment.
Learning about their condition and available interventions through educational programs equips patients with the knowledge to undertake treatments with confidence—even outside of a traditional medical facility.
Experts from The American Journal of Medicine stated that “Patient education significantly improves compliance with medication across a broad range of conditions and disease severities.
Conversely, lack of compliance is associated with poor clinical outcomes, increased hospitalizations, lower quality of life, and higher overall health costs.”
Yet, on average, healthcare professionals spend as little as six minutes training patients on the use of medication. And each time a patient withdraws from a trial due to non-compliance, it costs a sponsor 3 times as much to recruit a new patient.
Incorporating Patient Education Into Your Study Builds
The need for patient education is widely recognized in the research community. The increase in chronic illnesses, combined with limited hospital facilities, economic constraints due to U.S. healthcare, and a need for better and safer care at home greatly reinforce the importance of patient education.
Now, more than ever, clinical research needs to focus on building patient education into their study builds to boost retention rates and ensure the overall success of a study.
Improve Clinical Trial Awareness
Patient education can help ensure the people who want to participate in clinical research can source and identify a trial easily. The industry used to rely heavily on physician recommendations, but nearly half of millennials don’t have a regular primary care doctor. To improve awareness and education on clinical trial availability, teams can:
- Engage in training to raise awareness and knowledge of upcoming clinical trials with healthcare practitioners.
- Strengthen communication channels with healthcare professionals in a way that is clear, accurate, and jargon-free so they can pass that onward to their patients.
- Use plain language in clinical trial content and literature so patients understand it.
- Consider digital advertising to appeal to potential patients that don’t have primary care doctors.
- Engage with minority communities and bring clinical trial knowledge into the places minorities are seeking care.
- Encourage stakeholder participation in facilitating community-based participatory research (CBPR) to engage the community in research.
Establish Trust Early for Long-Term Patient Retention
Patient fear and anxiety during a trial can be easily remedied when trust is established with their healthcare and clinical team. Involve patients early in your development plan and establish modes for healthy two-way communication between patients and their research team.
This can be done by engaging with patient networks, hosting focus groups, conducting one-on-one interviews, or working directly with patient advisory boards or advocacy groups. You could even encourage former study participants and volunteer advocacy groups to share their clinical trial experience via social media, patient forums, and word of mouth to dispel myths and encourage new patients to come forward.
Establishing two-way communication between clinicians and trial participants is crucial for identifying the questions to ask and the outcomes to assess. It will create a more patient-centric trial workflow that leads to improved satisfaction for both stakeholders.
And pre-addressing patient concerns allows clinicians to trust their patients fully understand study participation requirements and have the measures in place to enable full adherence.
Educate on Physical and Financial Barriers
Although a greater emphasis has been given to understand the physical and financial barriers of clinical trials, they continue to result in widespread dropouts and under-enrollment.
Patients without proper knowledge of what’s expected in a trial may not consider routine care costs, time away from work for frequent clinical visits, travel to the site, lodging, meals, and even coordinating dependent care.
To prevent patient drop-out from misunderstanding what’s expected of them, educate your patients on the extent of the trial’s requirements. Prior to a study, start by conducting research to recognize and address the burdens in your trials.
Whenever possible, try to invest in decentralized, patient-centric technology that reduces the need for cumbersome site visits or manual patient-reported outcomes.
Provide patients with clear information about the potential trial-related burden and direct them to supportive resources like financial navigation or counseling. Making patients aware of the potential burdens from the onset increases adherence to trial requirements and lowers trial drop-out rates as patients plan for anticipated burdens.
Improve Informed Consent and Data Collection
The average number of eligibility criteria for patients increased 50% in the past 20 years. Data complexity impacts patient adherence and increases the trial burden due to the need for more site visits, procedures, and data collection.
Involving patients in your study build-out can help minimize the number of endpoints and produce data that is more relevant to your patients’ needs. In fact, patient input is helping researchers determine:
- How or when to collect data
- Data selection or assessment protocol
- When to reorder, shorten, or add data points
- Specific aspects of analytic approach (ex: suggest covariates)
- Interpretation of results
- Inform real-world use of results
- Ensure measure align with participants’ culture
Involving patients in your study build not only educates them on the steps involved in a trial, but it can also enhance the participant experience by lowering burden and putting a greater emphasis on patient-centric data collection.
To further increase patient adherence, build patient education into your consent form. You can achieve this by using plain language, patient-facing forms that speak directly to the patients’ needs, address their concerns, clearly outline the study goals, and are transparent about risks and commitments.
These steps enhance your global compliance while increasing comprehension and education in the process. Patient understanding is crucial to the success of your trial. Building patient education into your study plan can accelerate your builds while improving retention and strengthening your global compliance.
Participant Education Begins with Patient-Centric Technology
Patient education is the first step in adopting a patient-centric approach. But workflows are only as good as the solutions they are run on. Medrio’s unified platform, including EDC, eConsent, ePRO, RTSM, and DDC were purposefully designed with your patients in mind.
Replace clunky processes with our intuitive, drag-and-drop interface that allows you to build patient-friendly workflows without the need for programming or IT support.
Strengthen adherence with our feature-rich eConsent and an integrated eClinical suite that reduces the burden on the patient.
Are you ready to take the next step in your patient-centric journey?