Solutions > eCOA/ePRO
eCOA/ePRO Solution
Empower participants, clinicians, and observers. Enable convenient and accurate outcome reporting, enhancing data quality and study efficiency with electronic patient reported outcomes (ePRO) and electronic clinical outcome assessments (eCOA).
Increase Participant Engagement
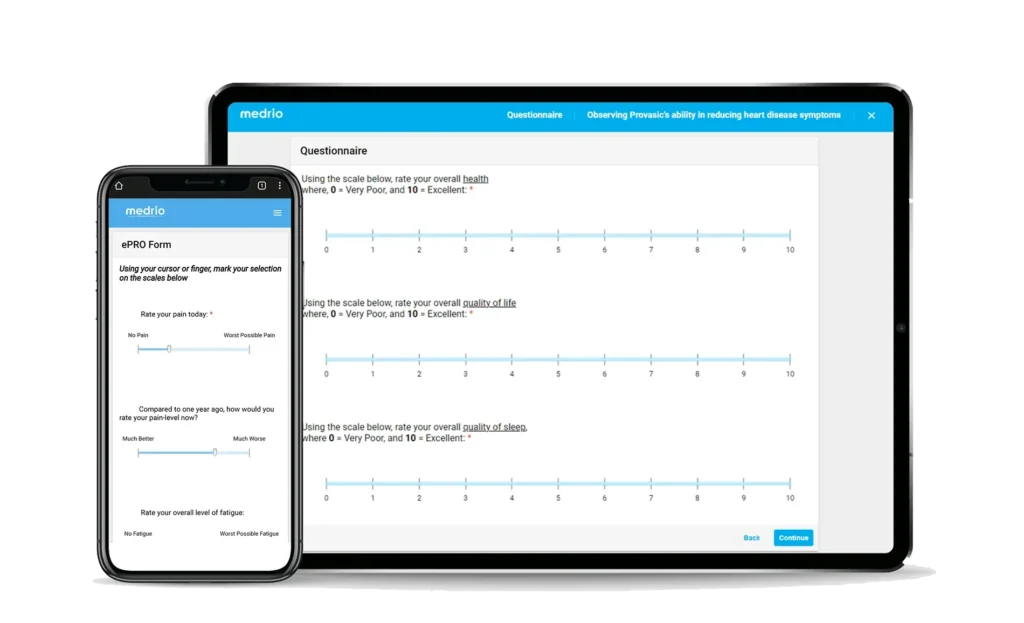
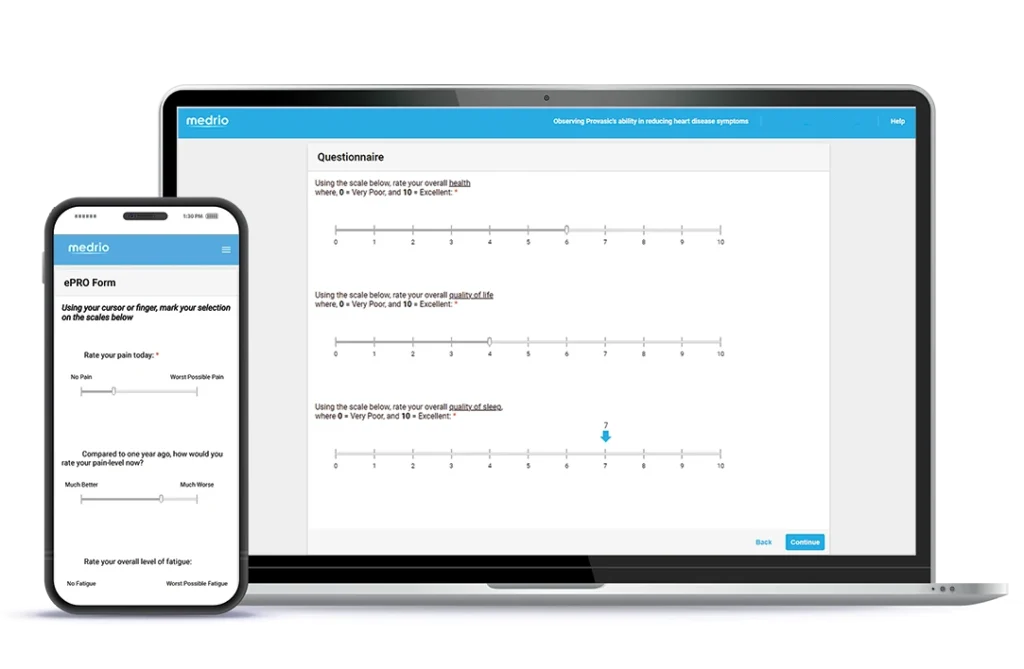
Participation is made easy with a web-based, device-agnostic solution.
Support Complex Protocols
Collect data in-clinic or remotely with validated and home-grown surveys.
Access Clinical Data in Real Time
View data to make real-time decisions with zero delays between collection and monitoring.
Improve Outcomes
Flexible Features for Faster Clinical Trials
Intuitive, configurable software built to improve the participant experience, site processes, and data quality from protocol to postmarket.
Stronger Clinical Evidence with Electronic Patient Reported Outcomes
Customer Testimonial
Accelerate Study Startup
Set up in as little as four weeks, accelerating the time it takes to begin capturing data. Quick, intuitive survey builds ensure you meet study timelines, ultimately reducing costs. Meanwhile, robust support helps you adhere to study protocols and industry standards.
What Medrio Customers Are Saying
Find out why Medrio has a 98% customer retention rate.
“Built with the builder in mind.”
“One of the easiest systems to implement mid-study updates. Queries are easy to find and respond to. Audit trails are straightforward as well.”
Dan Pontoriero
“The entire team is exceptional.”
“They are reliable and meet timelines. I got the same team members from beginning to end. Working with Ramana was a breath of fresh air!”
Claudia Ramos
SR. DIRECTOR, CLINICAL OPERATIONS
“Data simplified!”
“Medrio provides a system that is versatile and simplifies the complexities of data collection and management for administrators and data managers.”
Anonymous
OUR INTEGRATED SUITE OF SOLUTIONS
Maximize trial efficiency and operational excellence with intuitive clinical data management technology.
Improve data quality, engagement, and retention with a flexible, patient-centric solution.
Efficiently manage informed consent, enhancing compliance and participant experiences.
Drive success by partnering with an effective and strategic project management team.
Proudly Serving Life Science Innovators
Our commitment extends beyond providing solutions; it’s about being a part of a collective effort to drive positive change and innovation in critical sectors of the life science industry.
Frequently Asked Questions
What is eCOA and ePRO in clinical trials?
eCOA (electronic Clinical Outcome Assessments) and ePRO (electronic Patient-Reported Outcomes) are digital methods for collecting outcome data directly from clinical research participants, clinicians, or observers using electronic devices such as smartphones, tablets, or computers.
ePRO focuses specifically on patient-reported data, while eCOA can include clinician-reported and observer-reported assessments. Both replace paper-based solutions to improve data quality, accuracy, and timeliness.
What makes Medrio eCOA/ePRO a modern solution for clinical outcome assessments?
Medrio eCOA/ePRO software is built on Medrio’s unified clinical data platform, allowing outcome data to be collected and managed alongside clinical trial data in real time.
This solution offers an intuitive participant experience, flexible scheduling, device support, configurable surveys, and automated reminders to improve compliance and reduce site burden.
Is Medrio eCOA/ePRO an app?
In order to provide participants with the highest degree of flexibility and access, Medrio eCOA/ePRO is a web-based solution. This means that users can complete eCOA diaries in Medrio at any time, from any internet-connected device, without having to worry about device compatibility or operating system updates.
How does Medrio eCOA/ePRO improve patient engagement and compliance?
Medrio eCOA/ePRO enables participants to complete assessments remotely using familiar devices (known as Bring Your Own Device or BYOD), reducing the need for in-clinic visits. Automated notifications, reminders, and time-based schedules help participants stay on track, improving diary completion rates and overall data completeness.
How does Medrio eCOA/ePRO support data quality and regulatory compliance?
Medrio eCOA/ePRO captures outcome data electronically with built-in audit trails, time stamps, role-based access controls, and secure data transmission. These capabilities help support regulatory expectations for electronic records while reducing errors associated with manual transcription and paper diaries.
How does Medrio eCOA/ePRO integrate with Medrio EDC and CDMS?
Medrio eCOA/ePRO supports full integration with Medrio’s CDMS/EDC and broader clinical data management capabilities, creating a single, unified data environment. Outcome assessments flow directly into the study database, enabling real-time visibility, streamlined data review, and faster downstream analysis.
Can Medrio eCOA/ePRO support decentralized, hybrid, or complex trial designs?
Yes. Medrio eCOA/ePRO is well suited for decentralized and hybrid trials, supporting remote data collection alongside in-clinic assessments. Its flexible configuration also supports adaptive and complex study designs where outcome assessments may change over time.
How does Medrio eCOA/ePRO compare to paper or standalone ePRO systems?
Compared to paper, Medrio eCOA/ePRO improves accuracy, reduces missing data, and eliminates manual data entry. Unlike standalone ePRO systems, Medrio eCOA/ePRO is integrated with EDC and CDMS workflows, reducing reconciliation effort and simplifying clinical data management.
What types of validated assessments and instruments can Medrio eCOA/ePRO support?
Medrio eCOA/ePRO supports patient-reported, clinician-reported, and observer-reported assessments, including validated instruments and custom questionnaires aligned to protocol requirements.
For pharmaceutical clinical trials, Medrio eCOA/ePRO enables compliant collection of outcome data for primary and secondary endpoints while remaining flexible enough to support study-specific needs across sites and regions.
Does Medrio provide training and support for eCOA/ePRO implementation?
Yes. Medrio provides onboarding, training, and ongoing support to successfully implement Medrio eCOA/ePRO. Medrio’s team assists with study setup, assessment configuration, and operational best practices to ensure smooth adoption.
Learn more at the Medrio Community forum.
How does Medrio eCOA/ePRO contribute to overall clinical trial efficiency?
By unifying outcome assessments with clinical trial data, Medrio eCOA/ePRO reduces system fragmentation, accelerates study startup, improves data quality, and supports faster decision-making. This integrated approach helps teams gain clearer insights while lowering operational complexity.
Upholding the Highest Compliance Standards




