Solutions > eConsent
Electronic Consent (eConsent) Solution
Streamline all aspects of the informed consent process and enable participants to consent from anywhere — remotely or onsite.
Optimized Research Workflows
Capture paper or electronic informed consent forms (ICFs) simultaneously.
Assured Trial Compliance
EU-compatible and 21 CFR Part 11-compliant solution that meets ALCOA++ standards.
Increased Comprehension
Enhance participant comprehension and retention with images, video content, quizzes, and FAQ sections.
Unlock Efficiency
Simplifying Trial Operations Management
Automate participant consent requests and site document assignments, ultimately reducing site burden.
Empowering Flexible Study Workflows
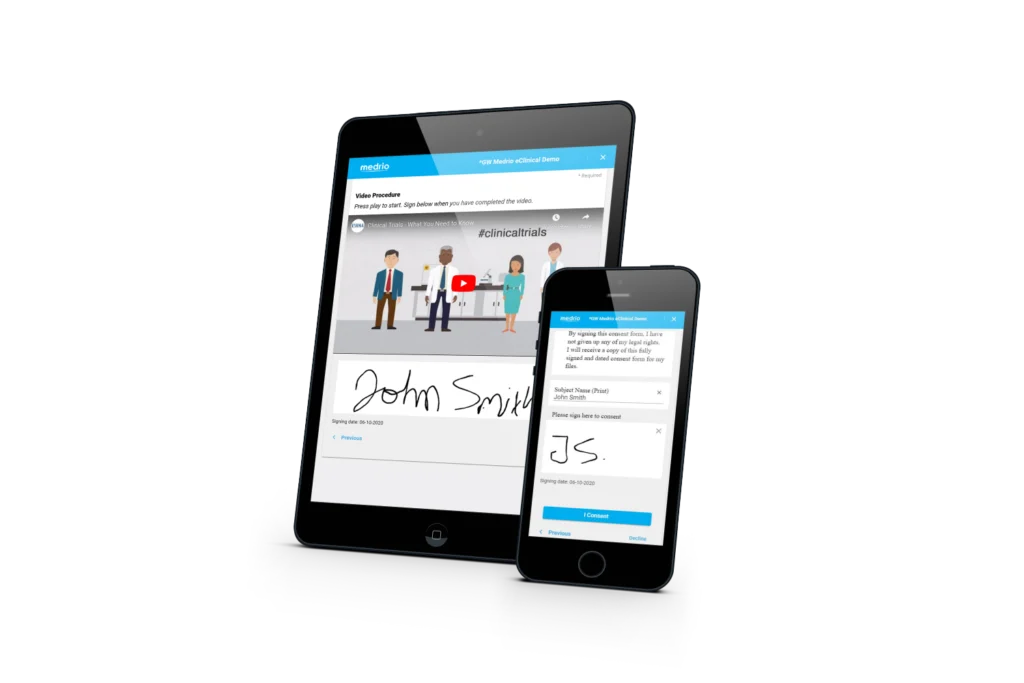
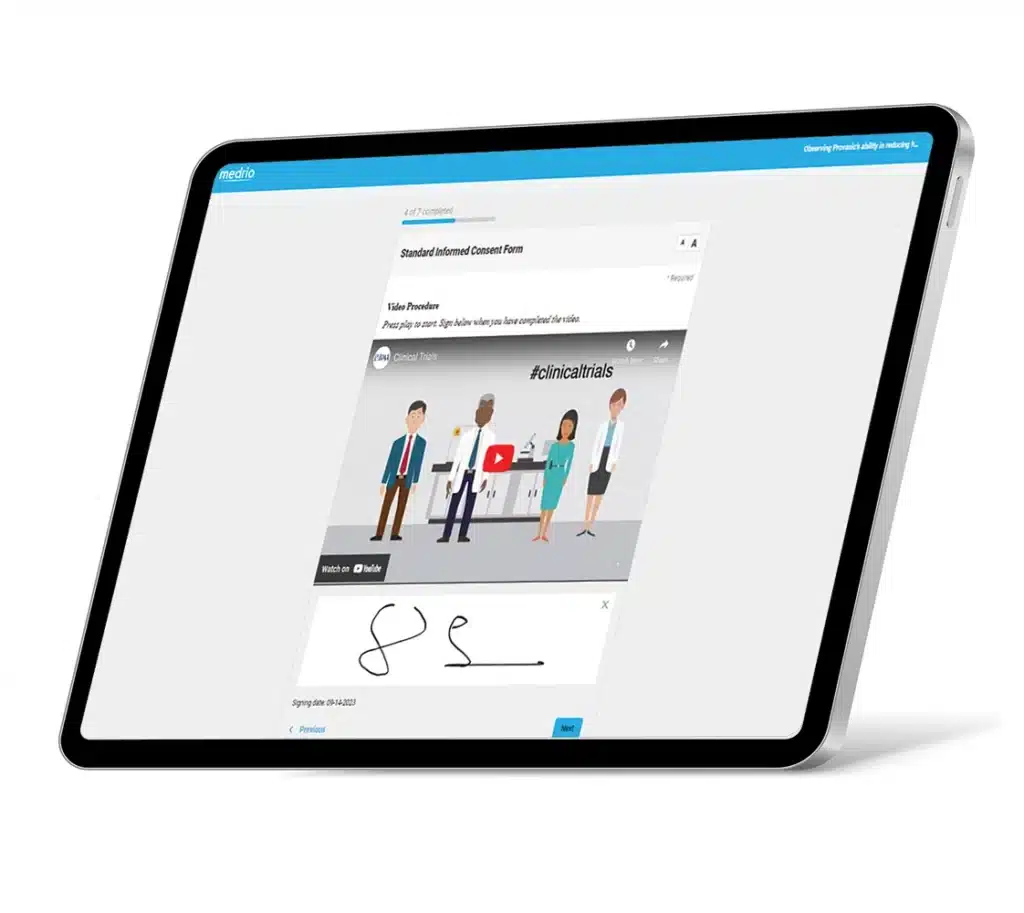
Our flexible, web-based solution supports electronic and paper-based processes, allowing you to prioritize the patients’ experience and the sites’ needs. Efficiently deliver consent forms; capture electronic signatures; and implement in-clinic, remote, or hybrid workflows.
Medrio econsent
Build a Better Informed Consent Process
Built-in flexibility means minimal workflow disruptions for sites and more autonomy for participants. You can upload paper consent, download and print unsigned consent forms, and collect eConsent across geographies with different regulations, making it easy to customize processes.
What Medrio Customers Are Saying
Find out why Medrio has a 98% customer retention rate.
“Built with the builder in mind.”
“One of the easiest systems to implement mid-study updates. Queries are easy to find and respond to. Audit trails are straightforward as well.”
Dan Pontoriero
“The entire team is exceptional.”
“They are reliable and meet timelines. I got the same team members from beginning to end. Working with Ramana was a breath of fresh air!”
Claudia Ramos
SR. DIRECTOR, CLINICAL OPERATIONS
“Data simplified!”
“Medrio provides a system that is versatile and simplifies the complexities of data collection and management for administrators and data managers.”
Anonymous
OUR INTEGRATED SUITE OF SOLUTIONS
Maximize trial efficiency and operational excellence with intuitive clinical data management technology.
Improve data quality, engagement, and retention with a flexible, patient-centric solution.
Efficiently manage informed consent, enhancing compliance and participant experiences.
Drive success by partnering with an effective and strategic project management team.
Proudly Serving Life Science Innovators
Our commitment extends beyond providing solutions; it’s about being a part of a collective effort to drive positive change and innovation in critical sectors of the life science industry.
Frequently Asked Questions
What is electronic informed consent (eConsent) in clinical trials?
Electronic Informed Consent (eConsent) is a digital approach to presenting, explaining, and documenting participant consent for a clinical study. It replaces traditional paper processes with an electronic form that participants can review, understand, and sign using a secure digital interface.
Medrio eConsent supports interactive content, electronic signatures, version control, and secure storage of consent forms, helping study teams improve participant understanding while maintaining compliance and traceability.
Why is eConsent important for clinical trials?
eConsent improves participation with multimedia elements, interactive explanations, and comprehension checks. These advantages enhance regulatory compliance, support remote consenting in decentralized studies, and provide a secure, traceable record of participant consent.
What makes Medrio eConsent modern and effective?
Medrio eConsent is intuitive, participant-centric, and fully integrated with Medrio’s clinical data platform. It supports interactive content such as videos and illustrations, dynamic consent flows, version control, and real-time data capture. This all helps study teams streamline the consenting process while improving participant engagement and retention.
How does Medrio eConsent support regulatory compliance?
Medrio eConsent captures consent electronically with time-stamped signatures, audit trails, role-based access, secure storage, and other best practices. These features help satisfy regulatory expectations for informed consent documentation and electronic case report forms (eCRFs) while ensuring traceability and inspection readiness.
Can Medrio eConsent be used for both in-clinic and remote consent?
Yes. Medrio eConsent supports flexible deployment models, allowing participants to review and sign consent documents either in the clinic or remotely using their own devices. This flexibility is especially valuable for hybrid and decentralized clinical trial designs that rely on virtual participation.
Can Medrio eConsent be used to manage paper-based consent processes?
For sites that require paper-based consent processes, Medrio eConsent can adapt. Users can upload wet-ink signed paper consent forms directly into Medrio eConsent’s digital workflow. This flexibility provides the benefits of digital consent storage and management even when paper-based consent is required.
How does Medrio eConsent integrate with EDC, CDMS, and other clinical systems?
Medrio eConsent is part of the unified Medrio clinical data platform, enabling seamless links between consent data and study data in Medrio CDMS/EDC. This integration ensures that consent status is visible to sites and study teams in real time and can be used to drive workflow logic and eligibility checks.
Does Medrio eConsent support versioning and re-consent?
Yes. Medrio eConsent allows study teams to manage consent document versions easily and to deploy re-consent when protocol changes require updated participant authorization. Version control and tracking help maintain compliance throughout the study lifecycle.
How does Medrio eConsent improve participant understanding and retention of key information?
Medrio’s eConsent supports multimedia content, such as videos, diagrams, and interactive quizzes that help participants better understand complex study concepts. These features can improve comprehension compared to paper forms and support informed participation.
What training and support does Medrio offer for eConsent implementation?
Yes. Medrio provides onboarding, enablement, and ongoing support for customers using Medrio CDMS/EDC. Customers receive study setup guidance, best practices for data management workflows, and responsive assistance throughout the study lifecycle to help teams use the platform effectively.
Learn more at the Medrio Community forum.
How does using Medrio eConsent benefit overall study operations?
Medrio eConsent streamlines the consenting process, reduces site workload, improves data accuracy, and accelerates study enrollment. Because it is integrated with Medrio’s broader clinical platform, it enhances real-time visibility into consent status, improves compliance, and supports smoother clinical trial execution.
Upholding the Highest Compliance Standards




