Medrio eCOA/ePRO
Empower participants, clinicians, and observers. Enable convenient and accurate outcome reporting, enhancing data quality and study efficiency with electronic patient reported outcomes (ePRO) and electronic clinical outcome assessments (eCOA).
Increase Participant Engagement
Participation is made easy with a web-based, device-agnostic solution.
Support Complex Protocols
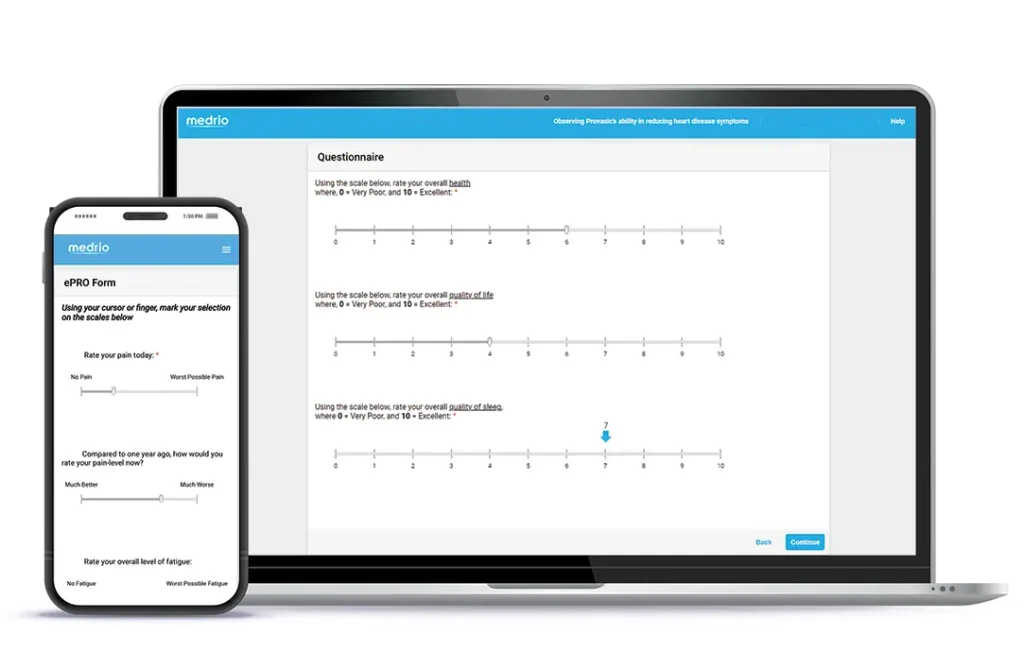
Collect data in-clinic or remotely with validated and home-grown surveys.
Access Clinical Data in Real Time
View data to make real-time decisions with zero delays between collection and monitoring.
Improve Outcomes
Flexible Features for Faster Clinical Trials
An intuitive, configurable solution built to improve the participant experience, site processes, and data quality from protocol to postmarket.
Stronger Clinical Evidence with Electronic Patient Reported Outcomes
Demonstrate value to regulators and payors with high-quality data. An easy-to-use interface, automated notifications, and the MyMedrio participant portal improve participant compliance and retention.
Customer Testimonial
Accelerate Study Startup
Set up in as little as four weeks, accelerating the time it takes to begin capturing data. Quick, intuitive survey builds ensure you meet study timelines, ultimately reducing costs. Meanwhile, robust support helps you adhere to study protocols and industry standards.
What Medrio Customers Are Saying
Find out why Medrio has a 98% customer retention rate.
“Built with the builder in mind.”
“One of the easiest systems to implement mid-study updates. Queries are easy to find and respond to. Audit trails are straightforward as well.”
Dan Pontoriero
“The entire team is exceptional.”
“They are reliable and meet timelines. I got the same team members from beginning to end. Working with Ramana was a breath of fresh air!”
Claudia Ramos
SR. DIRECTOR, CLINICAL OPERATIONS
“Data simplified!”
“Medrio provides a system that is versatile and simplifies the complexities of data collection and management for administrators and data managers.”
Anonymous
Upholding the Highest Compliance Standards





